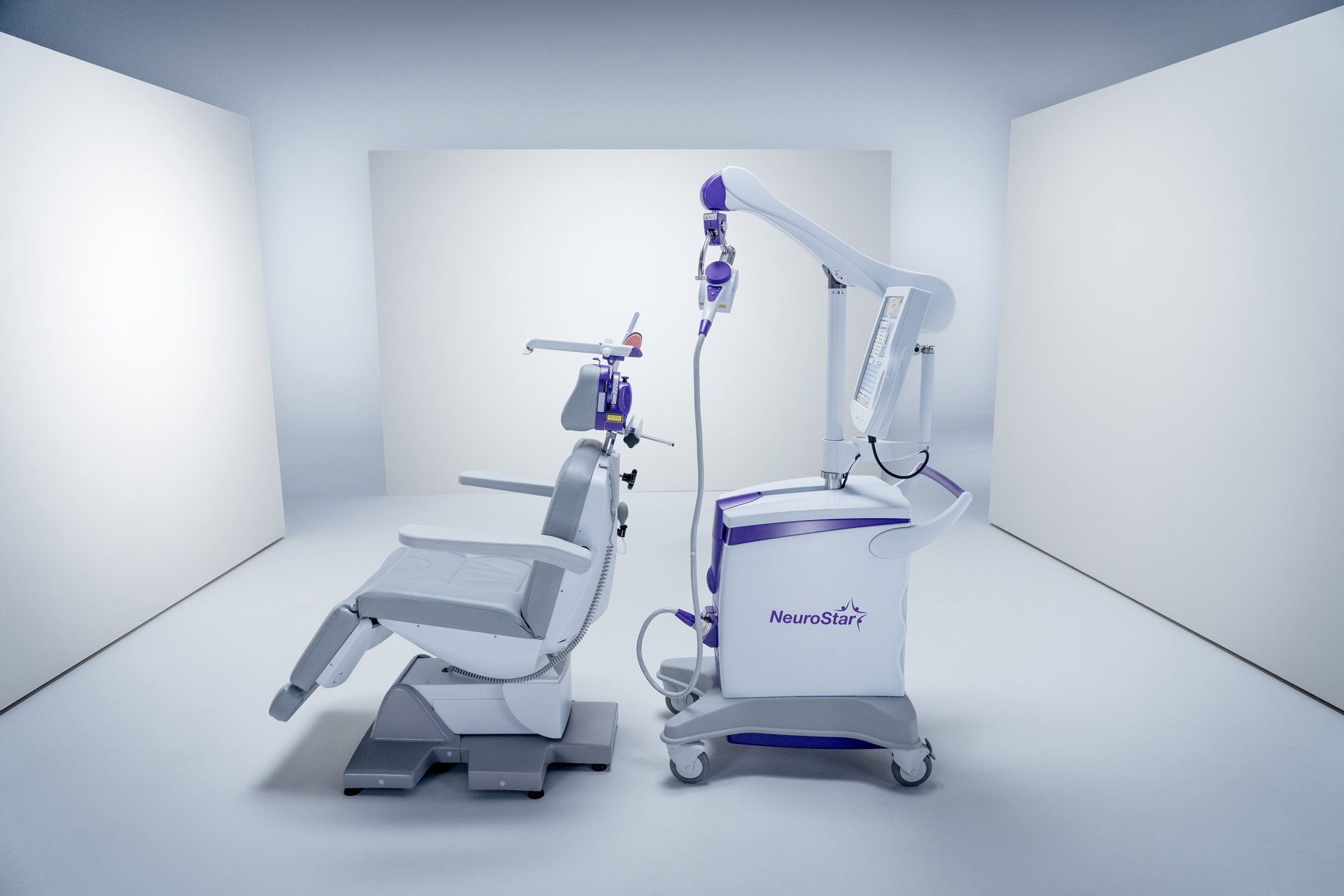
About NeuroStar TMS Therapy
NeuroStar utilizes transcranial magnetic stimulation (TMS) to stimulate specific areas of the brain that are less active in individuals with depression. Unlike electroconvulsive therapy (ECT), NeuroStar is a non-invasive treatment. While the exact cause of depression remains uncertain, the prevailing scientific theory suggests it results from an imbalance in neurotransmitters—chemical messengers responsible for communication between brain cells.

What is NeuroStar TMS Therapy?
NeuroStar treatment involves the use of a magnet with a strength comparable to that of an MRI machine to stimulate nerve cells in the brain regions associated with mood regulation. This stimulation may help restore neurotransmitter balance, offering the potential for long-term relief from depression.
NeuroStar TMS Therapy is:
- Administered in a doctor’s office
- Non-disruptive—patients can resume normal activities immediately
- Conducted while the patient is awake
- Free from adverse effects on memory or sleep
- Covered by most major insurance providers, including Medicare and Tricare
With over three million treatments performed, NeuroStar is offering renewed hope to individuals seeking effective relief from depression.
How NeuroStar TMS Therapy Works
Here’s what you can expect during a NeuroStar TMS Therapy Session:
Before Treatment
You’ll relax in a comfortable treatment chair while a small, curved magnetic coil is gently placed on your head.
During Treatment
NeuroStar delivers targeted magnetic pulses to specific areas of the brain. You may hear a clicking sound and feel a light tapping sensation on your head.
After Treatment
Each session lasts between 19 and 37 minutes, based on your doctor’s recommendation. Since NeuroStar does not affect memory or alertness, you can drive yourself home and resume daily activities right away. Treatment is typically administered in-office five days a week over a period of 4 to 6 weeks.*
TMS Testimonials
TMS Clinical Trials & Academic Studies
- Carpenter LL, et al. (2012). Transcranial Magnetic Stimulation (TMS) for Major Depression: A Multisite, Naturalistic, Observational Study of Acute Treatment Outcomes in Clinical Practice. Depression and Anxiety, 29(7):587-596. www.ncbi.nlm.nih.gov/pubmed/22689344
- George MS, et al. (2010). Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch Gen Psychiatry, 67(5):507-516. www.ncbi.nlm.nih.gov/pubmed/20439832
- Dunner DL, et al. (2014). A Multisite, Naturalistic, Observational Study of Transcranial Magnetic Stimulation (TMS) for Patients with Pharmacoresistant Major Depressive Disorder: Durability of Benefit Over a 1-Year Follow-Up Period. J Clin Psychiatry. 75(12):1394-1401. www.ncbi.nlm.nih.gov/pubmed/25271871
- O’Reardon JP, et al. (2007). Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol Psychiatry, 62(11):1208-1216. www.ncbi.nlm.nih.gov/pubmed/17573044
Frequently Asked Questions
Adult Indications for Use
The NeuroStar Advanced Therapy System is indicated for the treatment of depressive episodes and for decreasing anxiety symptoms for those who may exhibit comorbid anxiety symptoms in adult patients suffering from Major Depressive Disorder (MDD) and who failed to achieve satisfactory improvement from previous antidepressant medication treatment in the current episode. The NeuroStar Advanced Therapy System is intended to be used as an adjunct for the treatment of adult patients suffering from Obsessive Compulsive Disorder (OCD). Important Safety Information – NeuroStar Advanced Therapy is only available by prescription. A doctor can help decide if NeuroStar Advanced Therapy is right for you. Patients’ results may vary. The most common side effect is pain or discomfort at or near the treatment site. These events are transient; they occur during the TMS treatment course and do not occur for most patients after the first week of treatment. There is a rare risk of seizure associated with the use of TMS therapy (<0.1% per patient). Visit neurostar.com for full safety and prescribing information.